|
|

When It Comes To String Tension, Consistency Is The Name Of The Game
By Steve Crandall
Vice President, Sales & Marketing
Ashaway Racket Strings
Top pros are notorious for stringing their racquets to suit whatever conditions they will be playing in: if it's hot and humid, they'll string at one tension; cold and dry, they'll string at another. They do this to ensure that their racquets (and they always show up with a whole bag of them) will be at their precisely preferred tension when they get out on the court to play. They want consistency, but even so, stringers often hear complaints.
Most of us don't have the luxury of daily restringing, of course, or (ahem!) the talent to warrant it. But still, we like our shots to be crisp and predictable, and not have our racquets behave like butterfly nets. So, are we simply at the mercy of the weather, or are there other factors involved? What is it with this tension loss business, anyway?
First, understand that different string materials react differently to weather conditions. Nylon, for example, is hygroscopic: that is, it absorbs moisture, which adversely affects tension-holding properties. Think cooked spaghetti versus raw. Polyesters are somewhat less hygroscopic, but only polyketones are impervious to moisture.
However, temperature and humidity are not the only things that affect tension-holding properties. Polymer chemistry also plays a major role. To see just how big a role, USRSA Certified Master Racquet Technician and veteran stringer John Gugel of Racquet Quest recently ran some tests, the results of which he has graciously allowed us to present.
What John did was take three leading brand strings -- a polyester monofilament, a synthetic gut (nylon), and Ashaway MonoGut® ZX polyketone monofilament -- and strung them at 60 lbs. on identical Babolat Pure Drive GT racquets, using the same Wilson Baiardo stringing machine. He then stored the strung racquets under stable temperature and moisture conditions, and measured the string tension with a Beers ERT 300 every day for 21 days. None of the racquets were played with or disturbed in any way, so the tension loss data shown in the chart below represents purely the tension response of the different materials, isolated from all other effects.
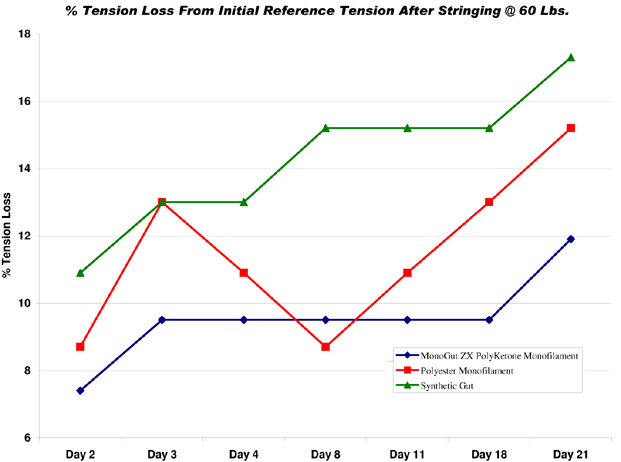
As you can see, all three strings lost tension in the first couple days after stringing as they "settled" into the frames. The polyester and synthetic gut both lost about 13% during this period, while the MonoGut ZX lost 9.5%. After that, the synthetic gut continued to lose tension fairly steadily, with a couple plateaus of stability in between. Interestingly, the polyester actually gained tension again until day 8, after which it lost it fairly rapidly, which might explain the pros' complaints about tension despite their restringing efforts. The MonoGut polyketone lost the least tension of all three strings, and held it steady the longest.
Overall, synthetic gut lost the most tension at 17.3%. Polyester was the second biggest loser at 15.2%, and was by far the most inconsistent. The MonoGut ZX polyketone lost least at 11.9%, and was most consistent throughout the trial. To put that another way, the polyester string lost nearly 30% more tension than the MonoGut ZX, and the nylon synthetic gut lost over 45% more.
So, why is this? To answer that we need to delve into the arcane mysteries of polymer science--although not too deeply because it's more confusing than the tax code. If you took organic chemistry, you might remember that a polymer is a compound comprised of repeating structural units, often called a backbone. (If you really want to impress your friends, you might quote from Wikipedia and say that "the term derives from the ancient Greek word  (polus, meaning "many, much") and (polus, meaning "many, much") and  (meros, meaning "parts")). (meros, meaning "parts")).
In any event, polymer backbones, or chains, consist mainly of carbon atoms, although other elements can also be included. These chains are held together with various types of bond. The simplest for our purposes, is a covalent bond, which is a chemical link between two atoms--in this case, carbon to carbon--in which the electrons are shared between them. An aromatic bond involves a more complex molecular structure that, according to Britannica, "includes one or more planar rings of atoms, usually but not always six carbon atoms. The ring's carbon-carbon bonds are a type characteristic of these compounds, in which electrons are shared equally with all the atoms around the ring in an electron cloud." In terms of tension holding properties, aromatic bonds are much stronger.
So, here's the deal: nylon (a polyamide) is a long chain carbon polymer with simple, covalent bonds. When used to make strings, it loses tension because the bonds break down. Polyester is a more complex polymer chain with one aromatic bond. It holds tension better than nylon, but as we saw, is very inconsistent. Polyketone is an even more complex chain with three aromatic bonds. Thus, it holds tension better, longer, and more consistently than other strings. And consistency wins.
|

